Opportunities in the unknown

Opportunities in the unknown
Jacob Corn is at the forefront of genome editing, developing technologies to cure genetic diseases and advancing research to gain a deeper understanding of human health.
What is your group working on at ETH?
JACOB CORN – One of our lines of research is to develop new genome-editing technologies that could one day cure genetic diseases. At the same time, we want to better understand how these interventions impact the human body. Most of the editing systems we work with modify an individual patient’s cells by altering specific parts of their genome. But every edit causes damage that the cells must then repair. This is why we want to discover how cells repair DNA damage so that we can develop editing systems that are safer and more precise. Another area we’re exploring is how cells break down and recycle their own components, particularly under stress or during ageing. These mechanisms play a central role in many chronic diseases and understanding how they work holds potential for future therapies.
© Adobe Stock / ETH Foundation
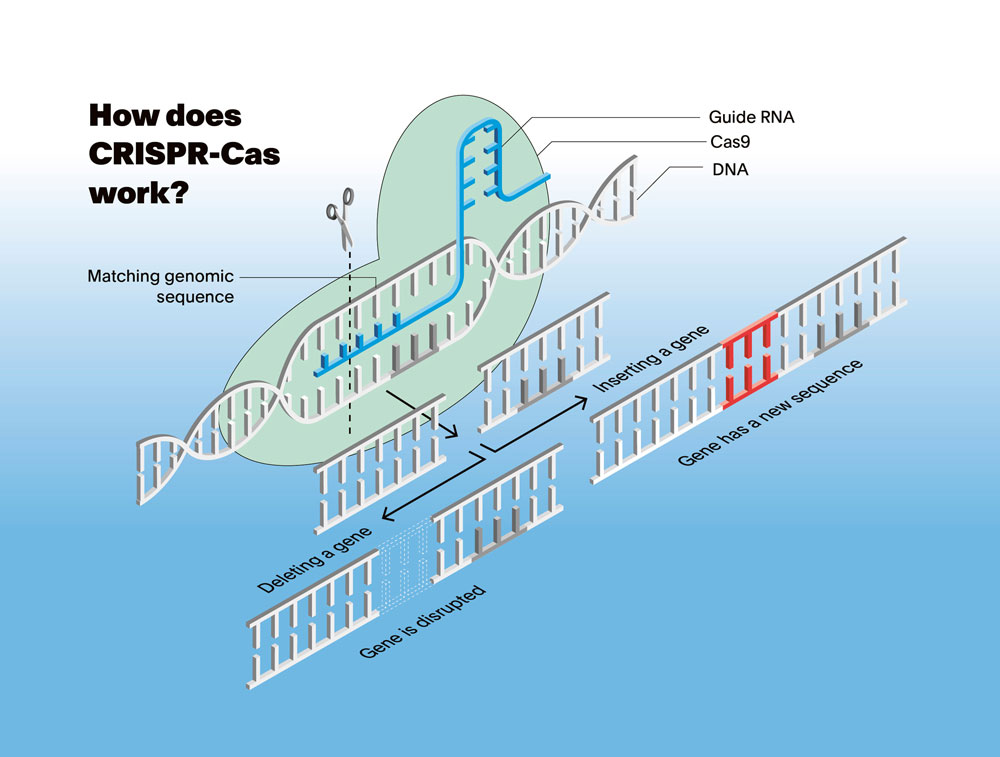
You’ve been a leading figure in genome biology for many years. How has the discovery of the CRISPR-Cas gene-editing tool changed your field?
I see the development of CRISPR-Cas as a symbol for how science can be democratised. Genome editing has been around since the 1990s but, back then, the systems were slow, expensive and limited to a small group of experts. Because CRISPR-Cas has simplified the technology so starkly, biologists, medical researchers and even students working on genetic diseases are now able to use the tools. This has created space for exciting new approaches and given a tremendous boost to our field. Focus is no longer set on just the technology itself but on how it’s used – whether it’s treating diseases or uncovering biological mechanisms we never knew existed. Of course, this also raises new ethical and safety concerns that must be carefully considered.
With both basic research and pharmaceutical solutions, you and your group cover a lot of ground. How important is basic research to you?
It’s crucial! After all, it forms the very foundation of applied research. The discovery of CRISPR-Cas is a good illustration of the vital role basic research plays in society, as it was from insights gained from fundamental research in the microbiological field that CRISPR-Cas emerged. As a result of these findings, a wide range of applications were developed within just a few years.
Which diseases are you focusing on in your applied research?
We’re developing treatments for hereditary blood diseases, such as sickle cell anaemia. While these conditions are rare in Europe, nearly 400 million people worldwide are affected. For me personally, the prevalence of a disease is not my key consideration. Many common conditions, such as bacterial infections, have multiple treatment options. By contrast, although genetic mutations can now be identified through DNA sequencing, we still lack cures. My vision is a future where children born with rare inherited diseases are not only diagnosed but actually cured.
How does applied research make its way from your lab into clinical practice?
One way is by founding a spin-off company. Besides spurring the development of promising therapies, it’s also good for the economy: a former doctoral student who launches a startup creates jobs and generates economic value. It’s great that ETH champions this entrepreneurial spirit through its Pioneer Fellowships. Thanks to this support, Jan Nelis and his company Immitra Bio are further developing a platform for gene therapies, while Lilly van de Venn is advancing her method to detect off-target effects in genome edits – unintended changes in the genome. Another important way is through collaborating with clinicians themselves. Doctors often come to us with specific medical questions, leading us to research new therapeutic approaches.
Why is ETH Zurich the right place for your research?
ETH offers an unparalleled blend of basic research and technological developments, all under one roof. This environment is incredibly inspiring. The university also brings together outstanding minds with state-of-theart infrastructure, is highly international and well connected. Its proximity to leading hospitals such as USZ and the Children’s Hospital, as well as to other excellent educational and research institutions, provides the perfect setting for our work.
Your professorship is funded by the NOMIS Foundation and the Lotte und Adolf Hotz-Sprenger Stiftung. What does this support mean to you?
The support we receive from foundations and donors through the ETH Foundation is invaluable. Grant funding is often tied to strict conditions, requiring researchers to outline expected results in advance. But groundbreaking discoveries happen when we explore the unknown with an open mind. Unrestricted philanthropic funding gives us the freedom to pursue precisely this kind of visionary research, allowing us to venture into truly new territory.
About
Jacob Corn’s career bridges science and industry, spanning therapeutic fields such as infectious diseases, neurobiology and oncology. After earning his doctorate in 2008 from the University of California, Berkeley, he began his research career as group leader at the biotech giant Genentech. He later returned to UC Berkeley as founding Scientific Director of the Innovative Genomics Institute and lecturer, before joining ETH Zurich in 2018.


